For cancer therapy, dozens of new therapies enter clinical trials every year, but in the end, less than 4% of the drugs are approved by the FDA. Although there are many influencing factors for this result, the main problem is that we do not fully understand how or why a particular cancer responds to treatment. Therefore, it is currently impossible to combine the right drugs in the best way and match them with the right patients .
However, the emergence of artificial intelligence may be able to help us. Most machine learning models are “black boxes”. They do not need to understand or pay attention to the biological mechanism of predicting results in order to optimize prediction accuracy.
Recently, researchers from the University of California, San Diego School of Medicine stated that they have created a new artificial intelligence (AI) system called DrugCell , which makes it possible to match tumors with the best drug combination. With DrugCell, after inputting the data about the tumor, the system will return the most well-known drug, the biological pathway to control the drug response, and the best drug combination. Related research results were published on ” Cancer cell “.
 Previously, researchers developed a Visible Neural Network (VNN) to simulate a simple eukaryotic cell-Saccharomyces cerevisiae. The system is able to accurately predict the impact of genetic mutations on cell growth responses, while identifying the most relevant molecular pathways driving these predictions.
Previously, researchers developed a Visible Neural Network (VNN) to simulate a simple eukaryotic cell-Saccharomyces cerevisiae. The system is able to accurately predict the impact of genetic mutations on cell growth responses, while identifying the most relevant molecular pathways driving these predictions.
On this basis, they created a VNN called “DrugCell” that can simulate the response of human cancer cells to therapeutic compounds. DrugCell combines the internal working principle of the model with the hierarchical structure of human cell biology, so that it can predict the response of any drug in any cancer and design an effective combination therapy.
The cellular drug response is a complex phenomenon that depends on biological and chemical factors . In order to capture these two determinants of drug response in an interpretable model, the researchers designed DrugCell as a neural network with two branches. The first branch is VNN , which is based on records in the human gene (GO) database. 2086 biological processes are modeled to simulate the hierarchical structure of molecular subsystems in human cells. Each of these subsystems ranges from those involved in small protein complexes (such as the b-catenin destruction complex) to larger signaling pathways (such as the MAPK signaling pathway) to those involved in overall cellular functions (such as glycolysis). The system is assigned a group of artificial neurons to represent the state of the subsystem. VNN uses a total of 12,516 neurons, which are distributed in six different layers.
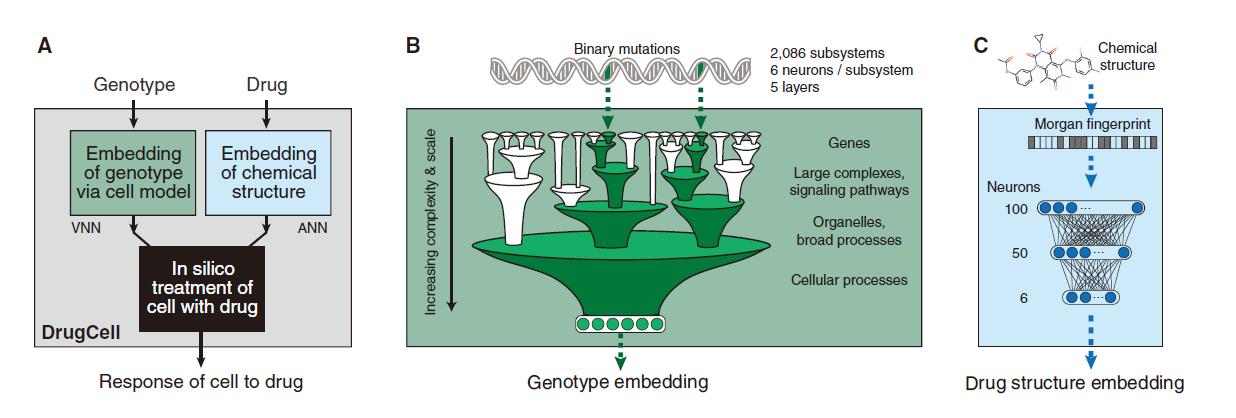
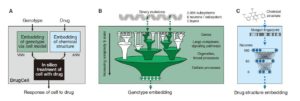
DrugCell design
The second branch of DrugCell is the traditional artificial neural network (ANN) , in which the Morgan fingerprint of the drug is embedded, which is the standard vector representation of the chemical structure. The output of the two branches in this model (VNN embedded in cell genotype and ANN embedded in drug structure) are merged into a single layer of neurons, and then integrated to produce a given genotype in response to a specific treatment. In addition, the model was trained on the response of 1,235 tumor cell lines to 684 drugs.
The results show that DrugCell can accurately predict the response of cell lines to treatment (the total accuracy of all cell line-drug pairs is: spearman correlation coefficient=0.80). In addition, the predicted combination improves the progression-free survival in patient-derived xenograft tumor models , and can stratify the clinical outcome of ER-positive breast cancer patients .

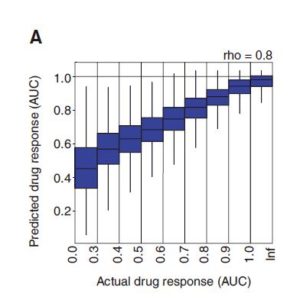
All researched (cell lines, drugs) are aligned to predict and actual drug response

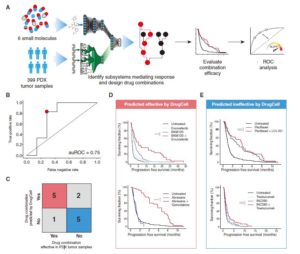
Joint guided treatment of patient-derived xenograft tumors
DrugCell has received training on the response of more than 1,200 tumor cell lines to nearly 700 FDA-approved drugs and experimental therapeutic drugs, and there are more than 500,000 cell lines/drug pairs in total.
First author Kuenzi said: “We are surprised by the ability of DrugCell to transform from laboratory cell lines into mouse and patient tumors and clinical trial data. But our ultimate goal is to get DrugCell into the clinic for the benefit of patients. Therefore, There is still much work to be done.”
The researchers also emphasized that although 1200 cell lines is a good start, it does not represent the complete heterogeneity of cancer . The research team is now adding more single-cell data and experimenting with different drug structures. They also hope to cooperate with existing clinical research to embed DrugCell into diagnostic tools and conduct prospective testing on it in reality.
References:
[1] Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells